Introduction
In regulatory affairs, there is an old saying, “If it’s not documented, it didn’t happen.”
In the context of producing government-regulated products, this principle means that every step in the development and manufacturing processes needs to be documented. If you decide to use an external supplier (for design, manufacturing, etc.), you need to write down the criteria you used to select them.
A Quality Management System (QMS) is used as a best practice to ensure all activities performed by the lab meet or exceed customer and regulatory requirements and conform to the organization’s pre-set policies and procedures. A QMS aims to capture the decisions you make (whether it’s about the design of a product, how a company operates, the manufacturing, etc.) and the rationale for those decisions.
Six components comprise proper electronic quality management systems:
- Document management: Documents are organized in a central database that includes audit trails and document workflows.
- Reporting management: Data is easily communicated.
- Inspection management: This component includes not only what an inspection will cover but also how the results of the inspection will be utilized to solve problems and improve the overall system.
- Audit management: Audits allow a company to “check off” that they are meeting all their regulatory requirements. Consultants are frequently brought in to perform audits to ensure impartiality.
- Learning management: Employees have to be trained on processes, and that training must be documented, so it’s essential to have good management of this education.
- Product lifecycle management: This component follows a product from ideation to market to disposal.
The QMS model follows 12 categories, as follows:
1. Organization
- The entire laboratory needs to be organized around consistent procedures and structures for monitoring ongoing quality.
2. Personnel
- Training, motivation, and engagement.
3. Equipment
- Every piece of equipment used in the laboratory must be maintained to operate safely.
4. Purchasing and Inventory
- Managing the supply chain is critical to ensure that raw inputs and other supplies are consistently high-quality.
5. Process Control:
- Quality Control (QC) processes for testing, including collection, handling, method verification, and process validation.
6. Information Management
- Data management is required to ensure all information is accurate, secure, confidential, and accessible to individuals with the appropriate privileges, such as lab managers and leadership.
7. Documents and Records
- Standard Operating Procedures (SOPs) must create a standard for each process.
- Documents need to be available at the point of work, and they must be maintained, accurate, and secure.
8. Occurrence Management
- A system must be in place to detect errors or non-conformances and facilitate investigations to discover the root causes and prevent reoccurrence.
9. Assessment
- Industry benchmarks (as well as QC managers, internal auditors, or external inspectors) are used to compare laboratory performance.
10. Process Improvement
- Continuous process improvement of laboratory processes with components like QC and CAPA (occurrence management).
11. Customer Service
- Operations should support a positive customer experience.
12. Facilities and Safety
- A set of procedures and standards to ensure a safe, secure, and clean environment (physically securing the lab, containment procedures for hazards, worker safety, etc.)
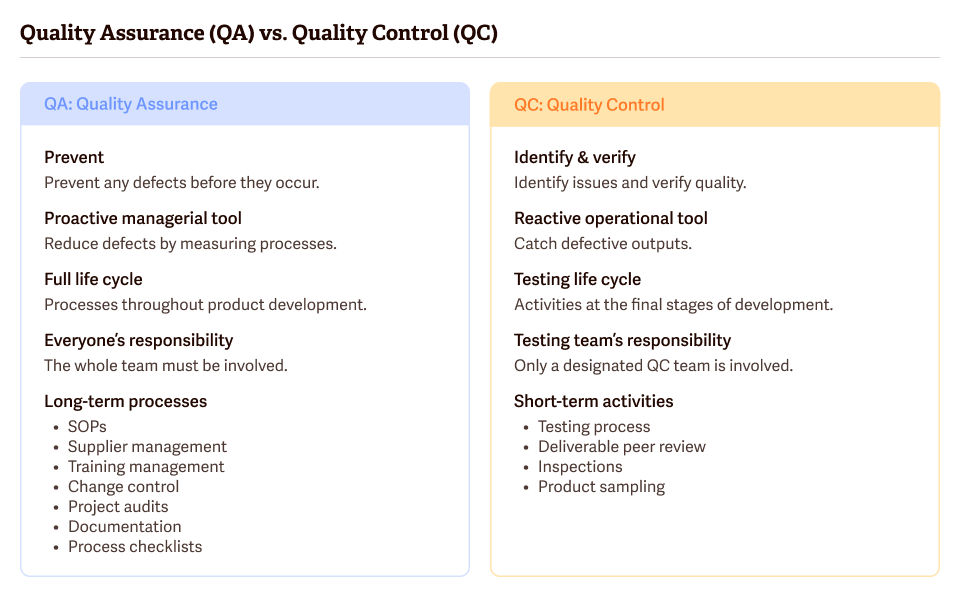
Quality Control and Quality Assurance
Quality Control (QC)and Quality Assurance (QA) fall under a subset of QMS.
What is Quality Assurance (QA)?
The ISO 9000:2015 standard, clause 3.3.6, defines QA as a set of planned activities within the product manufacturing process that ensure the product’s safety and quality. QA is the policies and procedures implemented before or during production that help prevent problems with the finished product.
What is Quality Control (QC)?
The ISO 9000:2015 standard, clause 3.3.7, defines QC as a systematic set of processes to ensure the product meets the required quality standards. QC means testing the processes involved in creating a product and testing the product itself to ensure the correct parameters are met. QC is a subset of QA.
QA and QC are both necessary for your QMS.
Quality indicators
Quality indicators should be used to make operational improvements. Quality indicators are criteria, standards, and other measures used to pinpoint performance and highlight problem areas.
Quality indicators must have these characteristics:
- Specific: Defines clearly the elements of the process measured, how data is collected, and what method was used to collect data.
- Objective and reliable: Gives consistent, evidence-based results across different data collectors.
- Measurable: Enables the analysis, recording, and evaluations (based on a benchmark) of operational processes.
- Relevant: Fits the purpose for which it was intended.
- Achievable: Allows for the accessible collection of data.
- Time-bound: Attaches to a time limit for when changes are expected and managed.
- Understandable: Enables all users to have a clear understanding of the indicator.
- Feasible and practical: Is simple and cost-effective.
- Useful: Can be used for decision-making.
Operational improvement models
Once you have a system for logging all of your decisions, you can optimize your operational processes.
One widely used operational improvement model is Six Sigma. The core purpose is to measure and eliminate defects in manufacturing processes until they are 99.9997% defect-free and focus on process and quality improvement and variation reduction.
Suppose you want to “reduce material waste by 50%.” Six Sigma’s DMAIC (Define, Measure, Analyze, Improve, Control) framework can help drive improvements.
Define: Where is the most material wasted?
The objective, “reduce material waste by 50%,” actually doesn’t adequately reflect what you need to be focusing on. Material waste is typically multi-factorial and highly complex—your company may have hundreds of waste products across a number of product categories.
You want to first identify the one waste product in the one product category that is leading to the most meaningful waste (perhaps the waste product costs the most or has the worst environmental impact).
Measure: How much material is being wasted here?
It’s possible that there isn’t a process flow diagram being utilized yet, and the inputs and outputs of each step within the process are not being tracked. Use a process map to identify waste, bottlenecks, and opportunities for improvement. Begin to identify patterns of events, problems, and defect causes.
Using the one waste product you selected, you want to begin to track the inflow and outflow (mass balances might make sense here) of this single waste product. How much and when is this product being wasted?
Analyze: Why is material wasted here?
You want to involve your team. You should be actively interviewing anyone involved with the waste material.
What is the root cause of the most waste? Is the waste product due to defects—if so, what kinds of defects, and why do the defects occur? Is the waste product due to limited expected shelf life—how long does the product last, and when is it being ordered?
Improve: What standards can we put in place to reduce waste?
What standards, protocols, software tools, etc., can you put in place to reduce waste?
If the waste product is due to limited shelf life, do you have an efficient inventory management system in place to reduce the amount of material you have in stock? How do you determine the quantity of material you order and will need during a specific period?
If the waste product is due to defects, are the product defects coming from an old piece of equipment that may be damaged and needs to be replaced or repaired? Do you need to set up a normal maintenance schedule? Do employees need to be better trained to use the equipment?
Control: Train your staff on these new standards.
Develop a set of protocols for these improvements, and assign someone on your team to ensure that everyone is adequately trained on these protocols.
There’s obviously a great deal of depth that you can go into at any step. There may be multiple waste streams and products that you could work on. Perhaps there’s no point-person assigned to the process, so there’s not a great deal of data on the waste product. There may be any number of standards and protocols that you can develop to improve waste reduction.
There are many other business improvement models that you can use, and we’ve included links to relevant resources at the end of the lesson:
- Total Quality Management (TQM): Aims to hold all parties involved in the production process accountable for the overall quality of the final product.
- Business Process Re-engineering (BPR): Focuses on designing workflows and business processes within an organization, not on the tasks themselves.
- Just In Time (JIT): Suppliers receive goods only as needed.
- Lean: Identifies and eliminates activities the end-user does not value. Aims to boost product quality, customer satisfaction, and employee performance.
- Theory of Constraints (TOC): Identifies the most critical limiting factor that stands in the way of achieving a goal and then systematically improves that constraint until it is no longer the limiting factor.
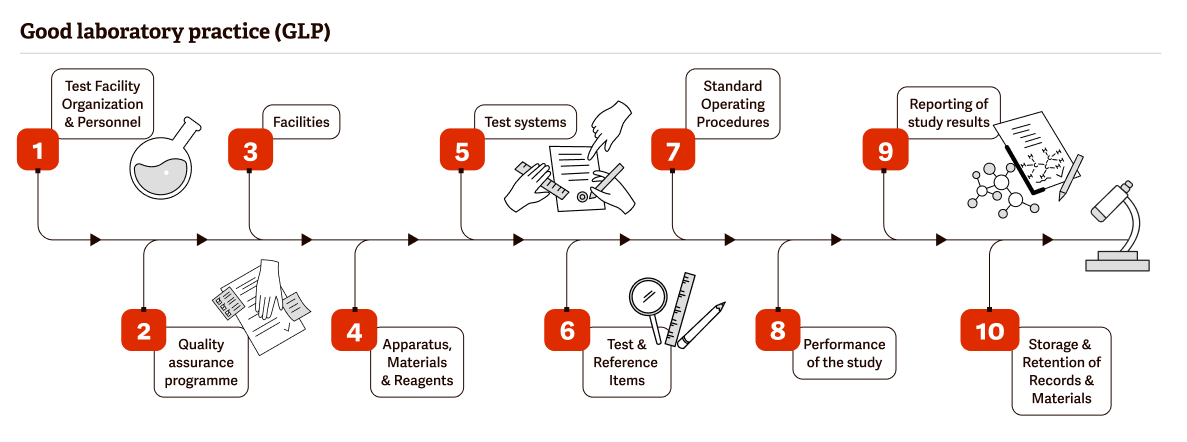
Good Laboratory Practice
Good Laboratory Practice (GLP) is a cornerstone of scientific quality testing and development.
GLP regulations were proposed to ensure that laboratories have a specific organizational structure, follow established scientific procedures, and record data in a way that protects integrity and ensures traceability. GLP monitors conditions, processes, documentation and archiving of studies and requires highly accurate and trackable data.
Without GLP, there is little chance of FDA or European Union approval of the resulting products.
Good Manufacturing Practice
Whereas GLP is concerned with research, Good Manufacturing Practice (GMP) is concerned with process. The purpose of GLP is to give regulators the information to scrutinize and audit the scientific validity of research studies. On the other hand, the purpose of GMP is to demonstrate that regulated products follow predefined criteria of manufacturing, processing, and packing.
Assessors include a review of the manufacturer’s compliance with the current GMP practices (cGMP), which shows that the company has the necessary facilities, equipment and ability to manufacture. Manufacturers must fulfill a set of quality requirements, such as lighting, plumbing, hygiene, storage, equipment maintenance, separation of substances to avoid contamination, quality raw materials, robust operating procedures, detection and investigation of product quality deviations, and lot release or lot conformance testing for products.
As the “C” in cGMP stands for “current,” companies are compelled to use the latest technologies and systems to follow the regulations. For example, equipment that may have been “top-of-the-line” to prevent contamination, mix-ups and errors 20 years ago may be less than adequate by today’s standards.
